More Information
Submitted: 14 February 2020 | Approved: 09 March 2020 | Published: 10 March 2020
How to cite this article: Lajis AFB, Ismail NH. Therapeutic application of herbal essential oil and its bioactive compounds as complementary and alternative medicine in cardiovascular-associated diseases. Insights Depress Anxiety. 2020; 4: 025-036.
DOI: 10.29328/journal.ida.1001016
Copyright License: © 2020 Lajis AFB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bioactive; Cardiovascular; Essential oil; Ischaemic heart; Pharmacologic effect
Therapeutic application of herbal essential oil and its bioactive compounds as complementary and alternative medicine in cardiovascular-associated diseases
Ahmad Firdaus B Lajis1,2* and Noor Hanis Ismail3
1Institute Bioscience, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
2Bioprocess Technology, Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
3Lot 1861, Belakang Pejabat Pertanian Lundang, 15200 Kota Bharu, Kelantan, Malaysia
*Address for Correspondence: Ahmad Firdaus B Lajis, Institute Bioscience, Bioprocess Technology, Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia, Email: [email protected]
Background: Herbal essential oil contains pharmacological benefits for intervention treatment of various diseases. Studies have demonstrated its antimicrobial, antioxidant, and anti-inflammatory effect involving in vitro cell culture and preclinical animal models. It has been also traditionally used to reduce anxiety and hypertension in human. However, scientific studies elucidating its mechanism of action and pharmacological targets, as well as its effectiveness and safety as phytotherapeutic compounds are still progressing. Recent studies showed its promising effect in depression-cardiovascular disease intervention. However, comprehensive evaluations to enlighten latest advancement and potential of herbal essential oil are still lacking.
Objective: In this systematic review, the depression-cardiovascular effects of herbal essential oil on lipid profile, biochemical and physiological parameters (e.g haemodynamic) are presented. The route of delivery and mechanism of action as well as main bioactive compounds present in respective essential oil are discussed.
Methods: Article searches are made using NCBI PubMed, PubMed Health, SCOPUS, Wiley Online, tandfonline, ScienceDirect and Espacenet for relevant studies and intellectual properties related to essential oil, depression and cardiovascular disease.
Results: In experimentation involving in vitro, in vivo and clinical trials, herbal essential oil showed its effectiveness in reducing coronary artery disease (narrowing of the arteries), heart attack, abnormal heart rhythms, or arrhythmias, heart failure, heart valve disease, congenital heart disease, heart muscle disease (cardiomyopathy), pericardial disease, aorta disease, Marfan syndrome and vascular (blood vessel) disease.
Conclusion: This review gives a valuable insight on the potential of essential oil in the intervention of depression associated with cardiovascular diseases. Studies showed that herbal essential oil could act as vasodepressor, calcium channel blocker, antihyperlipidemia, anticoagulant, antiatherogenesis and antithrombotic. It can be proposed as an interventional therapy for depression-cardiovascular disease to reduce doses and long-term side-effect of current pharmacological approach.
Depression is debilitating health mental disorder, a common and an independent risk factor associated with cardiovascular disease (CVD) and increased mortality. A deprived health condition in conjunction to the heart failure (e.g involving heart’s valve, pericardial, muscle) and malfunction of the blood vessels (e.g coronary artery, vascular) can ultimately lead to serious events such as heart attack or stroke. Increasing trend of morbidity and mortality linked to depression associated with cardiovascular disease has caused a great concern in many countries [1,2]. It also imposed financial burden to the government as well as hundred thousands of patients and their families [2-4]. It has caused considerable losses and reduced the quality of life of many individuals. In the last few years, it was estimated that more than 17 million of people died from CVD in United States and more than 1.5 million of people suffered from myocardial infarction [1,3,4]. In 2016, data from the Department of Statistics of Malaysia showed that ischaemic heart disease (e.g coronary heart disease, myocardial infarction) was the highest (15.3%) case of deaths among Malaysian as compared to other type of incidents or diseases [5]. The manifestation of CVD is often coupled with several risk factors such as family history, metabolic syndrome [e.g visceral obesity, glucose intolerance, insulin resistance, high triglyceride (TG), low high density lipoprotein (HDL)], hypertension and dietary composition in relation to atherogenesis and thrombosis [3,6]. Particularly, hypertension has become a prevalent risk factor for CVD morbidity whereby individuals with this risk factor was estimated to increase to about 60% with a total of about 1.6 billion people in 2025 [1-3]. Other risk factor such as coronary atherosclerosis is often pathologized by the accumulation of fats [e.g lipids, cholesterol and TG], within the arterial blood vessels, endothelial dysfunction as well as coagulation (e.g platelet-mediated) [7-9]. Important consequences of coronary atherosclerosis include coronary artery disease (CAD), angina (ischaemic chest pain) and myocardial infarction (MI). Many studies also showed that hyperlipidemia is the root cause of atherosclerosis, stroke and ischaemic heart disease whereby their correlation with single nucleotide polymorphisms of genes for lipid metabolism [e.g lipoprotein lipase (LPL) and apolipoprotein A5 (APOA5)] have been demonstrated [4,7,8]. Modern applications of pharmacological drugs (e.g Aspirin, statins, opioids) have their own limitations related to patient compliance, dose, effectiveness and side-effect mostly due to the differences in individual genetic makeup, foods, diets (e.g lipids ratio/type composition) and lifestyle [2, 10]. Several drawbacks from great reliance on modern pharmaceutical drugs has caused long-term problem on financial circumstances and health side-effect. Some CVD drugs have been implicated as causes of depression. Likewise, some antidepressant drugs (e.g Tricyclic antidepressants) are not suitable for CAD patient. Some other side-effects from the interaction between antidepressant and CAD drugs are also a concern in patient suffering depression with CAD. Noteworthy, patient with CVD is at an increased risk of developing depression. As high as 45% of patients with CAD (includes stable CAD), unstable angina or MI had experienced from clinically significant depressive symptoms. Hence, a new strategy to mitigate this disease using compounds of natural resources have been studied and proposed [8,10-14].
Research disclosed that marine fish, cod liver and plant oils with a high amount of polyunsaturated fatty acids (PUFA, e.g omega-3] reduced TG and LDL levels (e.g triglyceridemia), halt thrombosis (e.g platelet aggregation/reactivity, plasma viscosity) as well as alleviate atherosclerotic plaque formation and rupture thus preventing cardiovascular disease (e.g acute coronary syndrome). Hence, oils and lipids from such sources have been widely studied and reviewed [2,4,6,15]. Little attentions have been given to evaluate and review the beneficial depressive-cardiovascular function of oil of other resources. This is due to the fact that depressive-cardiovascular relevance of plant oils does not only rely on the presence of PUFA or their other functional lipids components (e.g phospholipids) [1,3]. Several analysis indicated that plant oils particularly herbal essential oils contain considerable amount of lipophilic bioactive compounds which are intervening CAD [13-17]. Several known flavonoids and other polyphenolic antioxidants [e.g ubiquinone, (vitamin E; tocopherols: tocotrienol), γ-oryzanol, ferulic acids triterpenyl esters] which were abundantly present in essential oils significantly inhibited oxidation of LDL-cholesterol and reduced thrombotic development [10-12]. These lipophilic antioxidants were capable of reducing thiobarbituric acid reactive substances (TBARS), lipid peroxidation (LPO), glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in vitro [18-20].
In these present years, several essential oils (EOs) have been demonstrated as alternative intervention therapies for both depression and cardiovascular disease. Recent advance in this new approach shows potential alternative to conventional dietary or pharmacological approach. EOs are unique as compared to vegetable/marine oils which the former contain many other distinctive bioactive compounds that potentially led to new discovery (e.g bioactive drugs or techniques) in the intervention of cardiovascular disease. In this present article, EOs and their bioactive compounds as potent complementary therapy in the intervention of depression associated with CAD are systematically reviewed using reliable sources and databases. Their pharmacological effects on lipid profile, biochemical and physiological (e.g heamodynamic) parameters are illustrated. This present article also elucidates mechanism of action and pharmacological targets of EOs, as well as its effectiveness and safety as phytotherapeutic compounds.
Search strategy and screening
Searches were performed using NCBI PubMed, PubMed Health, SCOPUS, Wiley Online, tandfonline, ScienceDirect and Espacenet databases. The records provide coverage of high quality and peer-reviewed articles in the fields of health and medicine. Searches were performed from January 2018 to February 2020 and were selected for review without any limitations. Terms and keywords relating to “essential oil” or “depression” and “cardiovascular” were used in the searches. Titles and abstracts were independently screened in accordance with our predetermined inclusion criteria. The reference lists of included studies were manually searched for potentially relevant studies. Studies that did not meet the inclusion criteria were excluded. Research topics were devised to essential oil and depression-CAD intervention. Review articles were excluded as well as studies examining the use of oil from non-plant origin. English and other language studies were included.
Data extraction
Data was collected based on author, date, intervention treatment (essential oils and bioactive compounds) and intervention results on depression and CAD. The outcomes were categorized and compared between studies with different essentail oils and their bioactive compounds.
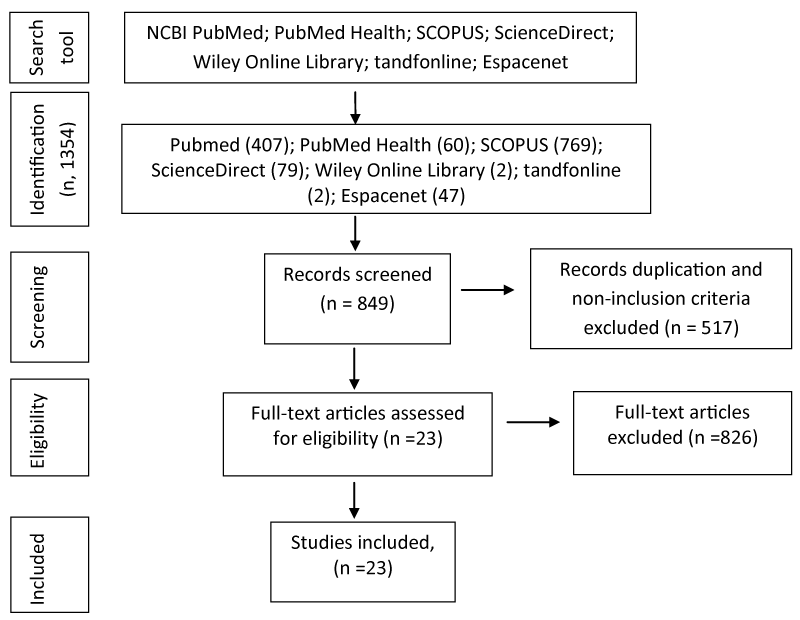
Searches in NCBI Pubmed and PubMed Health have retrieved works assessing the effect of essential oils and their bioactive compounds on CAD condition. In this systematic review, total of 1366 articles have been identified and 1269 of them have been excluded after screening of duplication and the titles/abstracts. The full-length research articles were retrieved in details and reviewed. A final total of 23 articles were selected for inclusion in this review. A flow-chart of the process of article selection is illustrated in figure 1.
Figure 1: Flow chart showing the process of selecting the relevant studies for the systematic review.
Several in vitro studies
Based on the search criteria, 90 selected articles were evaluated (Tables 1,2). in vitro studies indicated that EO of R. A. Tatarinowii. and R. A. Tatarinowii, had cytoprotective effect in cultured cardiomyocytes (e.g depress pulse frequency and increase the viability of cardiomyocytes) [21,22]. Particularly, R. Acori Tatarinowii EO ameliorated cell viability of neonate rat cardiac myocytes and reduced its pulse frequency [22]. In comparison, T. capitata EO had cytoprotective effect against LPO product (4-hydroxy-2-nonenal, pathophysiologic concentration, less than 10 µM)-induced neonatal rat cardiomyocytes death [23]. This EO at 20-40 parts per million (ppm) and pre-incubation at 1 h also reduced reactive oxygen species (ROS) generation and loss of mitochondrion membrane integrity but resembled cytotoxicity at concentration greater than 40 ppm [23]. The composition of the T. capitata EO revealed that it contained a considerable amount of monoterpenes [23]. In contrast, S. pinnatifolia EOs showed notable protective effect (high cell viability) on H2O2 induced death in rat myocyte culture in vitro [24]. Some other EOs such as F. A. zerumbet EO protected human endothelial cells from injury caused by oxidized low-density lipoprotein molecules. Its protective effect was mainly due to an increase of glutathione (GSH) and SOD activities [17,25].
| Table 1: Characteristics of the eligible studies. | |||||
| Author | Intervention herbs | Dose/duration | Route | Study sample | Result |
| Alves-Santos, et al. [36] | C. argyrophylloides | - | i.v | normotensive rats | ¯ Blood pressure |
| Ziaee, et al. [81] | L. angustifolia | 5-20 mg/Kg | i.v | isoproterenol-induced Myocardial infarction male rats | ¯ ST-segment elevation (ECG pattern) R-amplitude |
| Yan, et al. [24] | Syringa pinnatifolia Hems1. var. alashanensis | 8 -32 mg/kg | i.v | Wistar rats, Kunming mice | ¯ ADP-induced platelet aggregation ¯ Deviation of ST-segment, ¯ Creatine kinase, TnT, LDH, SOD |
| Bigliani, et al. [64] | S. areira | - | i.v | isolated mice hearts rabbits | ¯ systolic blood pressure |
| Ram, et al.[7] | Acacia senegal Seeds | 500 mg/kg/day | Oral | diet-induced atherosclerosis rabbits | ¯ atherosclerotic plaques in aorta lumen volume |
| Khan, et al. [59] | C. jwarancusa | - | i.v | ¯ body weight, lipid parameters ¯ blood sugar levels |
|
| Suanarunsawat, et al. [19] | O. sanctum L. Leaves | 2% fed | i.v | Male Wistar rats | ¯ high serum lipid profile ¯ atherogenic index |
| Kumar et al. [37] | P. elsholtzioides | In vitro | endothelium intact aortic prep | ¯ BP | |
| Rasheed, et al [50] | Rosa indica L. Petals | 0.01-3 ug/mL | i.v | Rabbit aorta rings prep | Calcium Channel Blocking Activity |
| Hosseini, et al. [68] | Lavender | 2%, 10 min | Olfactory | 40 open-heart surgery patients | ¯ blood pressure ¯ heart rate |
| Kim, et al. [66] | C. indicum Linné | - | Olfactory | subjects' | ¯ systolic blood pressure/ heart rate |
| Tahir, et al. [63] | Nigella sativa | 4-32 μL/kg | i.v | Rats | ¯ arterial blood pressure ¯heart rate |
| Lahlou, et al. [63] | O. gratissimum | 1-20 mg/kg | i.v | anaesthetized and conscious rats | ¯ mean aortic pressure ¯ heart rate |
| Shen, et al. [17] | A. zerumbet | 1-20 mg/kg | i.v | DOCA-salt hypertensive rats | ¯ mean aortic pressure |
| Santos, et al. [54] | H. fruticosa | 5-40 mg/kg | i.v | normotensive rats | hypotension |
| de Siqueira, et al. [32] | C. zehntneri | 10- 20 mg/kg | i.v | deoxycorticosterone-acetate (DOCA)-salt hypertensive rats | a vago-vagal reflex ¯ heart rate and blood pressure |
| de Siqueira, et al. [38] | Bark of A. canelilla | 1-20 mg/kg | i.v | normotensive rats | hypotension and bradycardia |
| de Menezes, [53] | C. winterianus EO | 1-20 mg/kg | i.v | Rats male Wistar | hypotension and vasorelaxation |
| Lahlou, et al. [42] | Mentha x villosa EO | 1-30 mg/kg | i.v | normotensive rats DOCA-salt-hypertensive rats |
Cardiodepressant peripheral vasodilation ¯ blood pressure |
| Note: TnT: Troponin-T; LDH: Lactate Dehydrogenase; SOD: Superoxide Dismutase | |||||
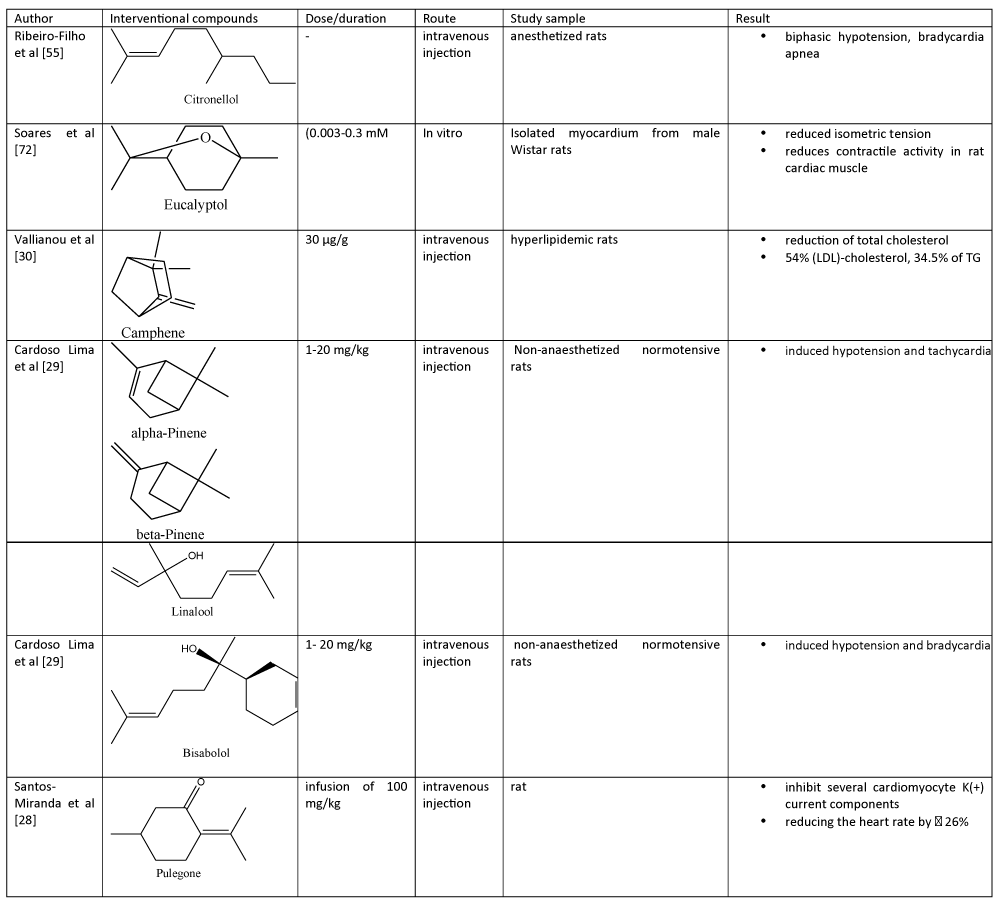
Table 2: Bioactive compounds in essential oils and their cardiovascular effects..
On the other hand, S. Chinensis Fructus EO and its bioactive compounds (e.g lignans, volatile oils and polysaccharides) have an ability to intervene several parameters in cardiovascular diseases mostly via antioxidant, apoptosis inhibition mechanism and anti-inflammation activity [26]. Study also showed that magnolol (C18H18O2), bioactive component from M. officinalis, up-regulated lipoprotein lipase (LPL) activity in a concentration-dependent, probably via alleviating LPL mRNA transcription, in mouse 3T3-L1 pre-adipocytes [27].
Similarly, R(+)-pulegone (C10H16), the main constituent of several EOs had antioxidant activity on isolated cardiac myocytes [28,29]. On the other hand, both A. melegueta and A. danielli seeds EOs reduced Fe2+-induced LPO in rats’ heart in a concentration-related manner [18]. They also inhibited angiotensin-converting enzyme (ACE) activity but lower than that of Captopril, a commercial drug for congestive heart failure [18]. GC-MS data identified eugenol (C10H12O2), 1,8-cineole (C10H18O), α-terpineol (C10H18O), α-caryophyllene (C15H24) and β-caryophyllene (C15H24) as major biocomponents in A. melegueta and A. danielli seeds EOs [18]. As compared to previously mentioned bioactive compounds, camphene (C10H16) which is abundant in Chios mastic gum EOs had anti-hypercholesterolemic and anti-hypertriglyceridemic effects on HepG2 cells with a significant reduction of cellular cholesterol level to a level comparable to that of mevinolin, a known 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor [30]. Unlike mevinolin, the former bioactive compound reduced cholesterol content via mechanism independent from HMG-CoA reductase activity [30]. Likewise, P. asiatica EO also modulated mRNA and protein expression of LDL receptor and HMG-CoA reductase in HepG2 cells [31].
Study using aortic ring preparations
Vasorelaxant effect in phenylephrine-induced contraction: Evaluations of cardiovascular disease intervention of EOs have also been conducted using isolated endothelium-containing aortic ring preparations pre-contracted with vasopressor drugs (e.g phenylephrine, prostaglandin F2α). In phenylephrine-induced contracted aortic rings, C. zehntneri EO, A. zerumbet EO, C. argyrophylloidesi EO and P. elsholtzioides EO exhibited vasorelaxant effect as observed in the presence or absence of 100 μM nitric oxide (NO) synthase blocker [Nitro-L-arginine methyl ester (L-NAME)] [32-37]. Particularly, C. argyrophylloidesi EO induced a dose-dependent aortic relaxation (IC50, ~25 μg/mL) that was associated with muscarinic receptor stimulation as well as liberation of the endotheliumderived prostacyclin whereby in experimented condition, its activity was greatly lowered by pre-treatment with anti-muscarinic agent (e.g 1 μM atropine, IC50 increase to ~197 μg/mL) [36]. Its action was also affected by cyclooxygenase blocker (e.g at 10 μM indomethacin, IC50 increase to ~91 μg/mL), vascular endothelium removal (at IC50 increase to ~76 μg/mL) and anti-diabetic agent (e.g at 100 μM glibenclamide, IC50 increase to ~64 μg/mL) [36]. Similarly, vasorelaxant effect of A. zerumbet EO was also dependent on endothelium and reduced in the presence of indomethacin and tetraethylamonium chloride [34,35]. It has also been reported that 1-Nitro-2-phenylethane, main constituent of A. Canelilla EO exerted vasorelaxant effect in endothelium-intact aorta preparations [38]. Nevertheless, as compared to C. argyrophylloidesi EO, some other EO was not antagonized by the presence of atropine. For instance, the vasorelaxant effect (IC50, 189 μg/mL) of C. zehntneri EO was not significantly altered by either removal of functional vascular endothelium or the treatment of 1 μM atropine [32]. C. zehntneri EO may induce a second and delayed hypotension due to its direct endothelium-independent vasorelaxant effects [33]. Likewise, vasorelaxant effect of eucalyptol was not dependent on endothelium [39]. On the other hand, in hypertensive rats-isolated thoracic aorta preparations, C. nepetaefolius EO (1-300 μg/ml) induced a doserelated reduction of phenylephrine-stimulated contraction [40]. Arteries from hypertensive rats had greater sensitivity to C. nepetaefolius EO, as noted by substantial reduction in its inhibition concentration [41].
Vasorelaxant effect in prostaglandin F2α -induced contraction: Meanwhile, in ~10 μM prostaglandin F2α-induced contracted aortic rings, both Mentha x villosa EO and Citrus aurantium EO induced vasodilatory effect (IC50, ~174) [42-48]. Comparatively, the vasorelaxant and hypotensive activity stimulated by Mentha x villosa EO and C. aurantium EO was also affected by endothelium removal while the former EO was also affected by the presence of 10 μM indomethacin (IC50, 334 μg/mL) [45,47]. Nevertheless, the vasorelaxant effect (IC50, 247 μg/ml) of latter EO was not affected by ~1 μM atropine but significantly reduced in the presence of nitric oxide (NO) synthase blocker (L-NAME) [47]. On the contrary, Mentha x villosa EO (255 μg/ml) exhibited vasorelaxant effect regardless in the presence or absence of L-NAME [45].
Vasorelaxant effect in potassium-induced contraction: In rat endothelium intact aorta preparations, A. Canelilla bark EOs (1-600 μg/mL) and C. zehntneri EO (1-1000 μg/mL) induced a dose-related reduction of K+ (60 mM)-stimulated contraction [IC50, ~65 and 202 μg/mL respectively)], an effect that was substantially reduced by treatment of high dose of atropine (10 μM) in the perfusion medium [IC50, 109.5 (72.5165.4) μg/mL)] [32,38,49]. In addition, C. argyrophylloidesi EOs -induced relaxation was also partly linked to the opening of potassium (K+) adenosine triphosphate (K-ATP)-channel which resulted in its hypotensive effect [36]. Mentha x villosa EO (10-500 μg/mL) and 1,8cineole (C10H18O) (0.006-2.6 mM) also antagonize the effect of potassium ions (60-80 mM) stimulated contractions (IC50, ~165 μg/ml) [39,42,45]. Cardiovascular effect of the Rosa indica L EO has been shown using isolated rabbit aorta preparations [50].
Electrophysiological evaluation elucidated that R. indica EO had greater potency against K+ (80 mM) as compared to phenylephrine precontractions [50]. Some major bioactive components were artemiseole (C10H16), isosteviol (C20H30O3), caryophyllene oxide (C15H24O), dihydromyrcene (C10H18), 5-octadecadien (C18H35NO), santolina epoxide (C10H16), and 9-farnesene (C15H24) as determined using GC-MS analysis [50].
Study using mesenteric artery preparations
On the other hand, Cymbopogon citratus, C. winterianus, H. fruticosa EOs and β-citronellol antagonize the effects of contractions induced by both or either phenylephrine or potassium in mesenteric artery rings [51-56]. Several bioactive compounds such as α-pinene (C10H16) and caryophyllene (C15H24), and 1,8 cineole (C10H18O) in H. fruticosa EO were suggested to promote its hypotensive effect [54]. Comparatively, vasorelaxant activity of C. citratus, C. winterianus and H. fruticosa EOs was not affected in denuded endothelium [52,54]. It was also suggested that hypotensive effect of C. citratus was not linked to K+ channels where no effect was observed in the presence of tetraethylammonium or potassium ions [52]. Meanwhile, β-citronellol (C10H210O) also suppressed spontaneous or electrical-evoked contractions of isolated left or right atrium of an adult rat [55]. A. speciosa EO reduced rat left atrial force of contraction with an IC50 of 292 μg/ml [57]. Compounds screening and identification via GC-MS analysis revealed that terpinen-4-ol (C10H18O, ~38%) and eucalyptol (C10H18O, ~18%) were high among 18 identified bioactive compounds in A. speciosa EO [57]. In another study, some bioactive compounds in EO such as carvone epoxide had higher relaxation effect on phenylephrine-induced contraction in mesenteric artery rings as compared to several other EO bioactive components such as limonene (C10H16), rotundifolone (C10H14O2), pulegone epoxide (C10H16O2), limonene epoxide (C10H16) and pulegone (C10H16) [29]. Apparently, molecular structures of previously mentioned bioactive compounds are functionally important in artery relaxation [29]. in vitro study will assist further analysis in vivo models to attain a deeper understanding and evaluation of EOs in cardiovascular disease.
Study using preclinical model
Cardioprotective effects against myocardium infarction: Several EOs have been used as a therapy for chest pain as well as in myocardial ischemia and myocardial infarction. Intraperitoneally administration of L. angustifolia EO (5-20 mg/Kg) with antioxidative property had cardioprotective effects in male Wistar rats with isoproterenol-induced myocardium infarction [81]. The previously mentioned EO ameliorated electrocardiogram (ECG) pattern by preventing ST-segment elevation and amplifying R-wave amplitude [81]. This is supported by the fact that L. angustifolia EO (1020 mg/Kg) notably reduced heart-body weight ratio and the increase of Malondialdehyde (MDA)-LPO and Myeloperoxidase (MPO)-neutrophils in myocardium and considerably lowered left ventricular end-diastolic pressure [81]. In experimental acute myocardium infarction (anterior interventricular branch of left coronary artery ligated with a 4/0 silk thread), administration of S. pinnatifolia EOs diminished deviation of ST-segment [24]. In biochemical parameters and serum marker enzymes analysis, it reduced level of related myocardial enzymes such as LDH, CK and Troponin-T with an increased in SOD activity as compared to myocardium infarction Male Wistar rats control [24]. Histopathological analysis showed that S. pinnatifolia EO had protective effect on myocardium infarction with lessen degree of necrosis and infiltration of inflammatory cells in rats [24]. Under hypoxia condition, S. pinnatifolia EOs (8-32 mg/kg) can prolong survival time of Kunming mice, suggesting its activity against hypoxia in experimental myocardium infarction [24]. Similarly, Nardostachyos Radix and S. pinnatifolia EOs exerted protective effect, thus preventing cell death- in chemical (e.g tert-Butyl hydroperoxide, H2O2)-induced injury in cardiomyocyte cultures (e.g H9c2, neonatal rat cardiac ventricular myocyte) [24,82]. The cell survival was higher with higher EO concentration due to significant reduction of ROS. EOs reduced the degree of myocardial infarction and the release of LDH and creatine kinase (CK), ameliorated the hemorheology index, increased SOD and glutathione peroxide activity in the myocardium and decreased MDA level [82]. Hesperetin had anti-apoptotic action on cardiomyoblasts via mitochondrion JNK/Bax pathway. Nobiletin activated the PI3K-Akt pathway, reduced cell apoptosis, and reduced myocardium infarct size, hence lowered the risk for myocardium ischemia and reperfusion injury [83,84]. These studies showed the effect of EOs as potent antimyocardial ischemia/infarction, and antimyocardial injury.
Hypolipidemic effect in rats and rabbits: In preclinical trial, Vallianou, et al. [30], and Abass, et al. [56], showed that EOs from Chios mastic gum had hypolipidemic effect in young and hyperlipidemic rats. Further evaluation indicated that camphene in this particular EO plays an important role in reducing the constitutive biosynthesis of serum cholesterol and TG [30]. Administration of its bioactive compound, camphene at a concentration of 30µg/g into hyperlipidemic rat resulted in diminished of total cholesterol (TC), LDL-cholesterol and TG to about 33% - 55% [30]. Similarly, administration of C. jwarancusa EO in experimental high-fat-carbohydrate diet rats reduced hyperlipidemic effect (e.g reduction in body weight, fats and blood sugar levels), thus potentially alleviate the risk of cardiovascular disease [59]. In a study, the administration of magnolol, reduced the serum lipid TG level (up to 50%) in hyperglyceridemic heterozygous transgenic mice (knock-in mice carrying APOA5 c.553G>T variant) [27]. On the other hand, O. sanctum EO reduced serum lipid profile (e.g TG, cholesterol) in normal and hypercholesterolemic Male Wistar rats [19]. Other EOs such as P. asiatica EO also exerted hypocholesterolaemic effect in C57BL/6 mice with significant reduction of plasma total cholesterol and TG (29% - 46%) as compared to untreated control [31].
Hypotensive effect on hemodynamic parameters in conscious rat and rabbit: In stark contrast, intravenous injection of A. zerumbet EO [includes its terpinen-4-ol and Eucalyptol], Croton zehntneri EO [includes its anethole and estragole], Ocimum gratissimum EO [includes its eugenol], Hyptis fruticosa EO, Mentha x villosa EO, S. areira EO, and Crocus sativus EO [includes its Safranal], A. Canelilla bark EO [includes its 1-Nitro-2-phenylethane] showed anti-hypertensive effect in rats [32-35,60,61]. However, hypotensive effect of A. zerumbet EOs was much lower than that of its pure bioactive terpinen-4-ol at same doses (1-10 mg/kg) [34,35]. In either experimented deoxycorticosterone-acetate (DOCA)-salt hypertensive or normotensive conscious rat, the effect of A. zerumbet EO, C. zehntneri EO, C. argyrophylloidesi EO, C. nepetaefolius EO (1 to 50 mg/kg), O. gratissimum EO, N. sativa oil, terpinen-4-ol and 1,8-cineole on hemodynamic parameters can be seen with reduction of mean aortic pressure, heart rate and arterial blood pressure [34,36,40,41,61-63] . These hypotension evidences on hemodynamic parameters of such EOs (e.g A. Canelilla, A. zerumbet, C. argyrophylloidesi and terpinen-4-ol) were mainly caused by active vascular relaxation in lieu to the withdrawal of nervous system sympathetic activity [34,36]. Similarly, the cardiovascular-hypotensive effect of O. gratissimum EO was most probably mediated independent of operational autonomic nervous system whereby its vasodilatory activity may have direct interaction with vascular smooth muscle [62]. In conscious rabbit, administration of S. areira EO reduced its systolic blood pressure, diastolic blood pressure, and mean arterial pressure in a pattern comparatively similar to nifedipine [64]. In hypertensive rats, pre-treatment with hexamethonium (30 mg/kg) decreased the bradycardia elicited by C. nepetaefolius EO (50 mg/kg) exclusively affecting the increment of C. nepetaefolius EO-stimulated hypotension [40,41]. This increment was linked to an increase in C. nepetaefolius EO- stimulated vascular smooth muscle relaxation with little evidence linked to the enhancement of sympathetic nervous system action in this hypertensive model (e.g its vasodilatory effects directly act upon vascular smooth muscle) [41]. Meanwhile, 1,8-cineole substantially reduced heart rate when only administrated at the highest dose (10 mg/kg) [35]. On the other hand, N. sativa and its thymoquinone (C10H12O2) had cardiovascular depressant effects, mediated primarily via indirect and direct mechanisms involving both 5-hydroxytryptaminergic and muscarinic mechanisms [63].
Likewise, intravenous administration of EOs bioactive compounds (e.g pinenes, citronellol, bisabolol and linalool) also produced hypotensive effect in conscious normotensive rats. Particularly, very high hypotension effect was noted by induction of β- pinene, citronellol and bisabolol at concentration of 20 mg/kg as calculated from its haemodynamics parameters (e.g mean arterial pressure and heart rate) [65]. Intravenous pre-treatment of conscious rats with hexamethonium (30 mg/kg) considerably reduced the resulted bradycardia produced from EOs administration (e.g A. Canelilla bark, O. gratissimum) without affecting their hypotensive effect [49,62]. Unlike O. gratissimum EO, the hypotension and bradycardia created by A. Canelilla bark EO were substantially decreased by pre-treatment with methylatropine (1 mg/kg) [62]. Similarly, cardiovascular depressant effect of N. sativa oil (4-32 μL/kg) or thymoquinone (0.2-1.6 mg/kg) on rats was substantially antagonized by certain concentration of cyproheptadine, atropine and hexamethonium [63]. Likewise, hypotensive and bradycardic responses evoked by Mentha EO in rats were blocked by pre-treatment with atropine (2 mg/kg) [42]. On the other hand, pretreatment with methylatropine (1 mg/kg) reduced bradycardic response without affecting hypotensive response [42]. In conscious rats, pre-treatment with hexamethonium (30 mg/kg), methylatropine (1 mg/kg) or atenolol (1.5 mg/kg) had no considerable effects on the 1,8cineole-stimulated hypotension, whereby bradycardic response to 1,8-cineole (10 mg/kg) was notably deceased by methylatropine [35]. These EOs possess the prospective of being an effective antihypertensive agent. Comparatively, P. elsholtzioides EOs contained high amount of bioactive compounds of sesquiterpenes and curzerene, benzophenone, α-cadinol and germacrone as analyzed by GC-FID and GC-MS [37]. These major compounds in this EO were suggested to play an important role in vasorelaxant and cardiovascular effects in Wistar rats whereby physiological and hemodynamic parameters indicated that this EO improved systolic and diastolic blood pressure, mean arterial pressure and heart beats after carotid artery cannulation [37].
Hypotensive effect on hemodynamic parameters in anesthetized rat: Concomitantly, EOs-derived β-Citronellol also had antihypertensive action with vasodilator effect [55]. In anesthetized rats, intravenous administration of β-citronellol led to biphasic hypotension, bradycardia and apnea [55]. In normotensive anaesthetized rat (e.g with pentobarbitone, urethane), the administration of 1,8-cineole (0.3-10 mg/kg), 1-nitro-2phenylethane (1-10 mg/kg) N. sativa (4-32 μL/kg) and O. gratissimum (1-20 mg/kg) elicited similar and concentration-dependent reduction in mean aortic pressure [35,38]. In anesthetized rats, injections of A. Canelilla bark EOs (1-20 mg/kg) generated concentrationrelated hypotension [38]. Pre-treatment of anaesthetized rats with bilateral vagotomy did not notably modify the O. gratissimum EO-stimulated concentration-related hypotension, while it considerably decreased the bradycardia at the highest concentration tested [62]. Mentha x villosa EO (1-500 μg/ml) and S. areira EO gave negative chronotropic (IC50: 229 μg/ml) and negative inotropic (IC50: 120 μg/ml) effects [64].
Clinical studies
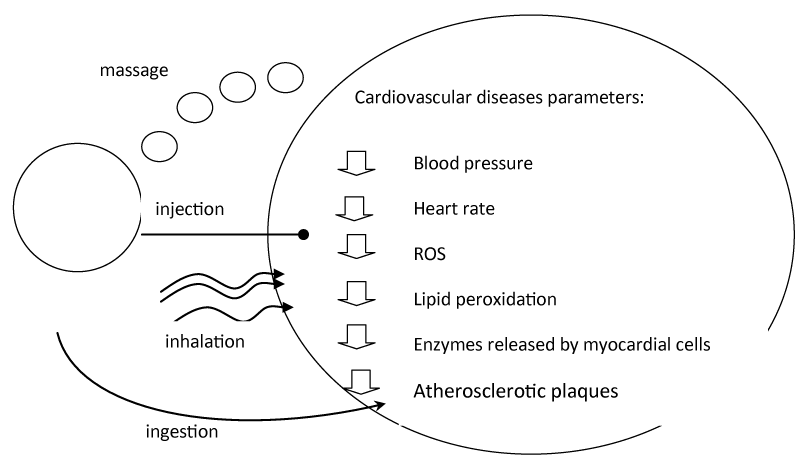
In clinical trial, it has been demonstrated that olfactory stimulation of the C. indicum Linné EO reduced systolic blood pressure and heart rate of the patients [66]. GCMS evaluation showed that 1,8-cineole and camphor as main biocompounds in this EO [66]. In a singleblinded randomized controlled trial, inhalation of EOs (e.g lavender and grapefruit) via olfactory stimulation (2% EOs for 10-20min) showed some repression on the inflated change values of diastolic blood pressure response in patients with stroke (with anxiety) and patients following coronary artery bypass and open-heart surgery [66-70]. While EOs alleviating stress and improved sleep quality in stroke patients, they had no noteworthy effects on mental stress and respiratory rate and other vital signs in patients underwent coronary artery bypass and open-heart surgery [69,70]. On the other hand, administration of A. calamus had significantly reduced chest pain, dyspnea, body weight index as well as improving ECG and lipid profile (serum cholesterol, LDL, HDL) in patients with ischemic heart disease [13]. Based on several studies, EO could be delivered via four different routes (Figure 2)./p>
Figure 2: Essential oil route of delivery.
Mechanism of action
Calcium (Ca2+) channel blocker: Cardiac Ca2+ channels (e.g T-type, L-type) in cardiac myocytes play functional role in heart, such as the resource of Ca2+-induced excitation-contraction and facilitate pacemaker depolarization of sinoatrial node in heart [8]. In particular, blockade of these channels has been used in the treatment of cardiovascular disease (e.g reduce contraction). Studies demonstrated that cardiodepressive effect of several EOs (e.g F. asafoetida, Citrus aurantium) and their bioactive components (e.g eucalyptol) rely onto their potential as Ca2+ channel blocker [47,48,71]. Particularly, eucalyptol depressed rate of force development by steady-state contractions, postrest potentiation, force development isometric force as well as positive inotropic effect created by Ca2+ [72]. F. asafoetida EO, A. speciosa EO, C. winterianus EO and Mentha x villosa EO had potent cardiodepressive and vasodilatory effect that mediated via endothelium-dependent (e.g EDRFs, NO and prostacyclin) and/or endothelium-independent mechanisms (e.g Ca2+ channel blockade) [57,71]. The previously mentioned EOs decreased the influx of Ca2+into the cell via plasma membrane Ca2+channels hence reduced the Ca2+-stimulated contractions [57]. Among EOs, A. speciosa EO probably have specific inhibition effect on L-type Ca²+ channels in rat heart [57]. The vasodilatory effect of F. asafoetida EO was decreased, but not fully inhibited, by either L-NAME or indomethacin [71]. H. fruticosa EO was also capable of antagonizing the dose-response curves to Ca2+ (3 μM-30 mM) in a concentration-related manner [54]. Concomitantly, the C. winterianus EO also antagonized the effect of Ca2+induced contractions in depolarizing potassium chloride solution which resulted in hypotension and vasorelaxation [53]. On the other hand, A. speciosa EO at 25 μg/ml and 250 μg/ml also inhibited left atrial force of contraction by up to ~33% and 89% respectively which was lower as compared to Nifedipine (a L-type Ca2+blocker) with an IC₅₀ of 12 μg/mL [71].
Anti-atherogenic effect: The effects of anti-atherogenicity of several EOs have also been demonstrated. The O. sanctum EO reduced atherogenic index as well as several enzyme/protein released by dying myocardial cells such as serum lactate dehydrogenase (LDH) and creatine-kinase (CK) activity with no adverse effect on high serum levels of aspartate/alanine aminotransferase and alkaline phosphatase in hypercholesterolemic rats [8,19]. EO also reduced elevated levels of TBARS, GPx and SOD with no adverse effect on catalase activity in the myocytes [19]. Histopathological evaluation indicated that EO was capable in preserving myocytes in experimental condition [19]. The compounds screening, identification and matching using GCMS revealed that eugenol and methyl eugenol as the major bioactive compounds in O. sanctum EO, thus suggested as being responsible for such pharmacological antihyperlipidemic effects [19]. In atherosclerotic rabbit model, treatment of A. senegal seeds (500 mg/kg/day) by ‘Per os’ for 45 d noticeably reduced serum level of TC, LDL-C, TG, and VLDL-C and atherogenic index (e.g with decreased atherosclerotic plaques in aorta, enlarged lumen volume) as compared to that of control [7]. The improvement on lipid profile and atherogenic index was noted almost comparatively similar to atorvastatin. Histopathological abnormality in aorta wall and other organs (e.g heart, kidney, liver) were reverted to normal condition with A. senegal seed supplementation, proven its antiatherosclerotic and cardioprotective effect.
Natural oil occurring lipophilic antioxidants (e.g. vitamin E, beta-carotene, flavonoids, monoterpene, terpenoids) inhibited the oxidative modification of low-density lipoproteins [73-80]. Studies showed that they could possibly prevent atherogenesis and coronary heart diseases. For instance, terpinolene which is the major compound in Pinus mugo EO as well as γ-terpinene from lemon oil effectively retard plasma low-density lipoprotein (LDL)-oxidation [78]. Upon treatment with terpinolene, it was found that the LDL (e.g lipid and protein part) was protected against copper-stimulated oxidation as indicated by conjugated dienes formation and delayed of loss of tryptophan peptide fluorescence [78-80].
Anti-coagulative, Anti-thrombotic and fibrinolysis effect: Heparin and coumarin derivatives are few of known anticoagulant compounds which help to reduce erythrocyte aggregation, platelet hyperactivity, arterial thrombosis and atherosclerosis, which were potentially useful to minimise the incidence of cardiovascular diseases (e.g coronary heart diseases myocardial infarction and cerebral arterial thrombosis) in human [85-92]. Particularly, Artemisia dracunculus leaves EO and F. Aurantii had a significant anti-coagulative effect [87,88]. The former had considerable amount of coumarin derivatives while the latter had significant amount of flavonoids which could regulate several coagulation parameters such as lengthening prothrombin time as well as reducing fractional shortening and left ventricular outflow, decreasing blood-clotting time in mice (e.g hematocrit and fibrinogen), and ameliorating the pathological alteration of myocytes in blood stasis model [87]. Some coumarins suppressed vitamin K-dependent γ-carboxylase action involved in the activation of coagulation factors [87]. Some citrus EO and its flavonoids (e.g hesperidin and hesperetin) inhibited the aggregation of erythrocytes and platelets. Hesperidin also inhibited ADP and thrombin-induced rat platelet aggregation [87]. Coumarins inhibited platelet functions via multiple mechanisms including scavenging of ROS and suppressing cyclic nucleotide phosphodiesterase and prostaglandin syntheses [87]. In addition, S. pinnatifolia EO at concentration of 1 to 5μg/mL also reduced adenosine 5’diphosphate (ADP) -induced rat platelet aggregation in vitro to about 33% - 47% [88]. In the anaesthetized Guinea-pigs, the C. bergamia Risso (bergamot) EO demonstrated a protective action against both pitressin-induced coronary arrhythmias and ouabain-induced ventricular arrhythmias [89]. In isolated heart of an adult rat, it also exerted a coronary-dilator action, reduced the hyperkinetic ventricular arrhythmias due to post-ischaemic reperfusion. The bergamot EO possesses significant cardiovascular effect comparable to that of an antidysrhythmic drug verapamil [89]. However, it is unknown whether it had similar action as verapamil by blocking voltage-sensitive Ca2+ L-type channels. It is well-understood that Ca2+ reduces after-depolarization and suppresses premature ectopic beats.
Anti-hyperlipidemia: Antihyperlipidemic activity of Pinus koraiensis leaves EO on several enzymes involved in lipid metabolism has been demonstrated using reverse transcription polymerase chain reaction (RT-PCR) and western blotting [90]. Molecular studies showed that P. koraiensis EO upregulated LDL receptor at the mRNA level and negatively inhibited the expression of several sterol regulatory element-binding proteins as well as HMG-CoA reductase, fatty acid synthase and G3P-acyltransferase in HepG2 cells [90]. It may binds to the active site of HMG-CoA reductase to reduce its activity. Consistently, P. koraiensis EO substantially reduced human acylcoenzyme A and cholesterol acyltransferases activity and suppressed the LDL oxidation. GC-MS analysis revealed that P. koraiensis EO consisted of camphene (~21%), d-limonene (~21%), α-pinene (~17%) and borneol (~12%) [90]. On the other hand, total blood cholesterol was reduced after C. citratus (lemongrass) EO-administration at the highest concentration tested [51].
Safety and limitation
Safe applications and doses of several EOs have been demonstrated [90-95]. For instance, clinical status (morbidity or mortality), morphology and lung:body weight ratio were unaffected by the administration of the EO S. areira [64]. On the other hand, LD50 of lemongrass EO based on a 24 h acute oral toxicity study in male Swiss mice was about ~3500 mg/kg with no significant changes in organs [51]. On the other hand, tachycardia was significantly observed in mice/rat administrated with pinenes at 20 μg/mL, citronellol at 5 μg/mL, and linalool at 20 μg/mL at increasing concentration while bisabolol at similar dose causes bradycardia (significant even at 5 μg/mL) [29,93-95]. On the other hand, high dose of C. winterianus EO stimulated transient bradycardia as well as arrhythmias due to a cardiac muscarinic activation secondary to a vagal discharge [53]. Meanwhile, administration of A. Canelilla bark EO elicited concentration-dependent bradycardia in rats. The bradycardia mostly depended in the presence of an operation-functional parasympathetic drive to the heart [38]. In conscious hypertensive rats, intravenous administration of C. zehntneri EO (1-20 mg/kg) stimulated rapid (2-4s) and concentrationdependent bradycardia [32]. The effect of bradycardia of C. zehntneri EO was reversed into tachycardiac effects by methylatropine (1 mg/kg) pre-treatment [32]. In contrast, administration of C. argyrophylloidesi EOs via intravenous administration in conscious rats created dose-dependent tachycardia [36]. In non-anesthetized normotensive rats, Hyptis fruticosa EO (5-40 mg/kg) also induced tachycardia [54]. In rats, pre-treatment of atropine, but not with atenolol or L-NAME, decreased tachycardiac responses by C. argyrophylloidesi EO [36]. However, hexamethonium pre-treatment converted the effect of the C. argyrophylloidesi EO-stimulated tachycardia into prevailing bradycardia [36]. Moreover, C. argyrophylloidesi EO-stimulated tachycardia in conscious rats may mediated via inhibition of vagal drive to the heart [36].
This systematic review demonstrated the therapeutic application of several EOs as complementary and alternative medicine in cardiovascular diseases. Terpenes are the most active components in EOs gave direct vasorelaxant effect as supported by the results of the present study. Other EOs lowered blood pressure, reduced risk for myocardial infarction, stroke and heart failure. This study provides a reference for the clinical research and utilization of EOs, as well as the application basis for co-treatment of cardiovascular diseases. This will also pave further evaluation of EOs for potential new application for cardiovascular diseases.
- Whayne TF. Ischemic Heart Disease and the Mediterranean Diet. Curr Cardiol Rep. 2014; 16: 491. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24743900
- Minihane AM. Fish oil omega-3 fatty acids and cardio-metabolic health, alone or with statins. Eur J Clin Nutr. 2013; 67: 536–540. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23403872
- Medina-Remón A, Tresserra-Rimbau A, Pons A, Tur JA, Martorell M, et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr Metab Cardiovasc Dis. 2015; 25: 60– 67. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25315667
- Devarajan S, Singh R, Chatterjee B, Zhang B, Ali A. A blend of sesame oil and rice bran oil lowers blood pressure and improves the lipid profile in mild-to-moderate hypertensive patients. J Clin Lipidol. 2016; 10: 339–349. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27055965
- World Health Organization - Noncommunicable Diseases (NCD) Country Profiles. 2018.
- Chicco AJ, Sparagna GC, McCune SA, Johnson CA, Murphy RC, et al. Linoleate-Rich High-Fat Diet Decreases Mortality in Hypertensive Heart Failure Rats Compared With Lard and Low-Fat Diets. Hypertension. 2008; 52: 549–555. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18663155
- Ram H, Jatwa R, Purohit A. Antiatherosclerotic and Cardioprotective Potential of Acacia senegal Seeds in Diet-Induced Atherosclerosis in Rabbits. Biochem Res Int. 2014; 2014. 436848. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25544897
- Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology. Elsevier Health Sciences. 2013.
- Qadir MI, Manzoor A, Akash MSH. Potential role of medicinal plants for antiatherosclerosis activity. Bangladesh J Pharmacology. 2018; 13: 59.
- Dai L, Lu A, Zhong LL, Zheng G, Bian Z. Chinese Herbal Medicine for Hyperlipidaemia: A Review Based on Data Mining from 1990 to 2016. Curr Vasc Pharmacol. 2017; 15: 520-531. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28707604
- Istvan ES, Deisenhofer J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science. 2001; 292: 1160–1164. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11349148
- Ali SI, Gopalakrishnan B, Venkatesalu V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review: Achillea millefolium L.: A review. Phytother Res. 2017; 31: 1140–1161. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28618131
- Rajput SB, Tonge MB, Karuppayil SM. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine. 2014; 21: 268–276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24200497
- Yu X, Sun S, Guo Y, Liu Y, Yang D, et al. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J Ethnopharmacol. 2018; 220: 265–282. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29628291
- Lajis AFB. Realm of Thermoalkaline Lipases in Bioprocess Commodities. J Lipids. 2018; 2018: 22. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29666707
- Bagheri S, Ahmadvand H, Khosrowbeygi A, Ghazanfari F, Jafari N, et al. Antioxidant properties and inhibitory effects of Satureja khozestanica essential oil on LDL oxidation induced–CuSO4 in vitro. Asian Pac J Trop Biomed. 2013; 3: 22–27. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23570012
- Shen X, Tao L, Li W, Zhang Y, Luo H, et al. Evidence-based antioxidant activity of the essential oil from Fructus A. zerumbet on cultured human umbilical vein endothelial cells’ injury induced by ox-LDL. BMC Complement Altern Med. 2012; 12: 174. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23039037
- Adefegha SA, Olasehinde TA, Oboh G. Essential Oil Composition, Antioxidant, Antidiabetic and Antihypertensive Properties of Two Afromomum Species. J Oleo Sci. 2017; 66: 51–63. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27928138
- Suanarunsawat T, Devakul Na Ayutthaya W, Songsak T, Thirawarapan S, Poungshompoo S. Antioxidant Activity and Lipid-Lowering Effect of Essential Oils Extracted from Ocimum sanctum L. Leaves in Rats Fed with a High Cholesterol Diet. J Clin Biochem Nutr. 2010; 46: 52–59. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20104265
- Verma SK, Jain V, Singh DP. Effect of Greater cardamom (Amomum subulatum Roxb.) on blood lipids, fibrinolysis and total antioxidant status in patients with ischemic heart disease. Asian Pacific J Trop Dis. 2012; 2: S739–S743.
- Danesi F, Elementi S, Neri R, Maranesi M, D’Antuono LF, et al. Effect of cultivar on the protection of cardiomyocytes from oxidative stress by essential oils and aqueous extracts of basil (Ocimum basilicum L.). J Agric Food Chem. 2008; 56: 9911–9917. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18928294
- Wu QD, Yuan DJ, Wang QW, Wu XR. Effects of volatile oil of Rhizoma Acori Tatarinowii on morphology and cell viability in cultured cardiac myocytes. Zhong Yao Cai. 2009; 32: 242-245. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19504971
- Hortigón-Vinagre MP, Blanco J, Ruiz T, Henao F. Thymbra capitata essential oil prevents cell death induced by 4-hydroxy-2-nonenal in neonatal rat cardiac myocytes. Planta Med. 2014; 80: 1284–1290. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25203731
- Yan Y, O W, Zhao X, Ye X, Zhang C, et al. Effect of essential oil of Syringa pinnatifolia Hemsl. var. alashanensis on ischemia of myocardium, hypoxia and platelet aggregation. J Ethnopharmacol. 2010; 131: 248–255. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20600760
- Huang N, Xu Y, Zhou H, Lin D, Zhang B, et al. Essential Oil from Fructus Alpiniae Zerumbet Protects Human Umbilical Vein Endothelial Cells in vitro from Injury Induced by High Glucose Levels by Suppressing Nuclear Transcription Factor-Kappa B Signaling. Med Sci Monit. 2017; 23: 4760–4767. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28976943
- Zhang MY, Wu HW, Xu LP, Yang HJ. Pharmacological effect of Schisandrae Chinensis Fructus and relative active components on cardiovascular and cerebrovascular diseases. Zhongguo Zhong Yao Za Zhi. 2018; 43: 1536–1546. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29751698
- Chang CK, Lin XR, Lin YL, Fang WH, Lin SW, et al. Magnolol-mediated regulation of plasma triglyceride through affecting lipoprotein lipase activity in apolipoprotein A5 knock-in mice. PLoS One. 2018; 13: e0192740. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29425239
- Santos-Miranda A, Gondim AN, Menezes-Filho JER, Vasconcelos CML, Cruz JS, et al. Pharmacological evaluation of R(+)-pulegone on cardiac excitability: role of potassium current blockage and control of action potential waveform. Phytomedicine. 2014; 21: 1146–1153. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24912864
- Cardoso Lima T, Mota M, Barbosa-Filho J, Viana Dos Santos M, De Sousa D. Structural relationships and vasorelaxant activity of monoterpenes. DARU. 2012; 20: 23. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23351149
- Vallianou I, Peroulis N, Pantazis P, Hadzopoulou-Cladaras M. Camphene, a Plant-Derived Monoterpene, Reduces Plasma Cholesterol and Triglycerides in Hyperlipidemic Rats Independently of HMG-CoA Reductase Activity. PLoS One. 2011; 6: e20516. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22073134
- Chung MJ, Woo Park K, Heon Kim K, Kim CT, Pill Baek J, et al. Asian plantain (Plantago asiatica) essential oils suppress 3-hydroxy-3-methylglutaryl-co-enzyme A reductase expression in vitro and in vivo and show hypocholesterolaemic properties in mice. Br J Nutr. 2008; 99: 67-75. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17697428
- de Siqueira RJ, Duarte GP, Magalhães PJ, Lahlou S. Cardiovascular effects of the essential oil of Croton zehntneri leaves in DOCA-salt hypertensive, conscious rats. Nat Prod Commun. 2013; 8: 1167–1170. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24079196
- de Siqueira RJ, Magalhães PJ, Leal-Cardoso JH, Duarte GP, Lahlou S. Cardiovascular effects of the essential oil of Croton zehntneri leaves and its main constituents, anethole and estragole, in normotensive conscious rats. Life Sci. 2006; 78: 2365–2372. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16325210
- Lahlou S, Interaminense LF, Leal-Cardoso JH, Duarte GP. Antihypertensive effects of the essential oil of Alpinia zerumbet and its main constituent, terpinen-4-ol, in DOCA-salt hypertensive conscious rats. Fundam Clin Pharmacol. 2003; 17: 323–330. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12803571
- Pinto NV, Assreuy AM, Coelho-de-Souza AN, Ceccatto VM, Magalhães PJ, et al. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine. 2009; 16: 1151–1155. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19524416
- Alves-Santos TR, de Siqueira RJ, Duarte GP, Lahlou S. Cardiovascular Effects of the Essential Oil of Croton argyrophylloides in Normotensive Rats: Role of the Autonomic Nervous System. Evid Based Complement Alternat Med. 2016; 2016. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27956919
- Shiva Kumar A, Jeyaprakash K, Chellappan DR, Murugan R. Vasorelaxant and cardiovascular properties of the essential oil of Pogostemon elsholtzioides. J Ethnopharmacol. 2017; 199: 86–90. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28132862
- de Siqueira RJ, Macedo FI, Interaminense Lde F, Duarte GP, Magalhães PJ, et al. 1Nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, elicits a vago-vagal bradycardiac and depressor reflex in normotensive rats. Eur J Pharmacol. 2010; 638: 90–98. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20406629
- Lahlou S, Figueiredo AF, Magalhães PJ, Leal-Cardoso JH. Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol. 2002; 80: 1125–1131. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12564637
- Lahlou S, Leal-Cardoso JH, Magalhães PJ. Essential oil of Croton nepetaefolius decreases blood pressure through an action upon vascular smooth muscle: studies in DOCAsalt hypertensive rats. Planta Med. 2000; 66: 138–143. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10763587
- Lahlou S, Leal-Cardoso JH, Magalhães PJ, Coelho-de-Souza AN, Duarte GP. Cardiovascular effects of the essential oil of Croton nepetaefolius in rats: role of the autonomic nervous system. Planta Med. 1999; 65: 553-557. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10483378
- Lahlou S, Ferreira Lima Carneiro-Leão R, Leal-Cardoso JH. Cardiovascular effects of the essential oil of Mentha x villosa in DOCA-salt-hypertensive rats. Phytomedicine. 2002; 9: 715–720. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12587691
- Guedes DN, Silva DF, Barbosa-Filho JM, de Medeiros IA. Endothelium-dependent hypotensive and vasorelaxant effects of the essential oil from aerial parts of Mentha x villosa in rats. Phytomedicine. 2004; 11: 490–497. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15500259
- Lahlou S1, Carneiro-Leão RF, Leal-Cardoso JH, Toscano CF. Cardiovascular effects of the essential oil of Mentha x villosa and its main constituent, piperitenone oxide, in normotensive anaesthetised rats: role of the autonomic nervous system. Planta Med. 2001; 67: 638–643. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11582542
- Guedes DN, Silva DF, Barbosa-Filho JM, de Medeiros IA. Endotheliumdependent hypotensive and vasorelaxant effects of the essential oil from aerial parts of Mentha x villosa in rats. Phytomedicine. 2004; 11: 490–497. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15500259
- Kang P, Ryu KH, Lee JM, Kim HK, Seol GH. Endothelium- and smooth muscle-dependent vasodilator effects of Citrus aurantium L. var. amara: Focus on Ca2+ modulation. Biomed Pharmacother. 2016; 82: 467–471. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27470386
- Suntar I, Khan H, Patel S, Celano R, Rastrelli L. An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid Med Cell Longev. 2018; 2018: 1–12. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29854097
- Rasheed HM, Khan T, Wahid F, Khan R, Shah AJ. Chemical Composition and Vasorelaxant and Antispasmodic Effects of Essential Oil from Rosa indica L. Petals. Evid Based Complement Alternat Med. 2015; 2015: 1–9. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26357519
- Costa CA, Bidinotto LT, Takahira RK, Salvadori DM, Barbisan LF, et al. Cholesterol reduction and lack of genotoxic or toxic effects in mice after repeated 21-day oral intake of lemongrass (Cymbopogon citratus) essential oil. Food Chem Toxicol. 2011; 49: 2268–2272. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21693164
- Moreira FV, Bastos JFA, Blank AF, Alves PB, Santos MRV. Chemical composition and cardiovascular effects induced by the essential oil of Cymbopogon citratus DC. Stapf, Poaceae, in rats. Revista Brasileira de Farmacognosia. 2010; 20: 904– 909.
- de Menezes IA, Moreira IJ, de Paula JW, Blank AF, Antoniolli AR, et al. Cardiovascular effects induced by Cymbopogon winterianus essential oil in rats: involvement of calcium channels and vagal pathway. J Pharm Pharmacol. 2010; 62: 215–221. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20487201
- Santos MR, Carvalho AA, Medeiros IA, Alves PB, Marchioro M, et al. Cardiovascular effects of Hyptis fruticosa essential oil in rats. Fitoterapia. 2007; 78: 186–191. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17343989
- Ribeiro-Filho HV, de Souza Silva CM, de Siqueira RJ, Lahlou S, dos Santos AA, et al. Biphasic cardiovascular and respiratory effects induced by β-citronellol. Eur J Pharmacol. 2016; 775: 96–105. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26872991
- Bastos JF, Moreira IJ, Ribeiro TP, Medeiros IA, Antoniolli AR, et al. Hypotensive and Vasorelaxant Effects of Citronellol, a Monoterpene Alcohol, in Rats. Basic Clin Pharmacol Toxicol. 2009; 106: 331–337. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20002067
- Santos BA, Roman-Campos D, Carvalho MS, Miranda FM, Carneiro DC, et al. Cardiodepressive effect elicited by the essential oil of Alpinia speciosa is related to L-type Ca2+ current blockade. Phytomedicine. 2011; 18: 539–543. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21112750
- Abass IS, Al-Ezzi MI, Arif IS, Jasim GA. Determination of Essential Oil Percentage with Evaluation of Antihyperlipidemic Activity of Three Natural Gums in Rats. 2010; 4.
- Khan SJ, Afroz S, Khan RA. Antihyperlipidemic and anti-hyperglycemic effects of Cymbopogon jwarancusa in high-fat high-sugar diet model. Pak J Pharm Sci. 2018; 31: 1341–1345. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30033418
- Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective Effect of Saffron Extract and Safranal in Isoproterenol-Induced Myocardial Infarction in Wistar Rats. Iran J Basic Med Sci. 2013; 16: 56–63. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23638293
- Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, et al. The Effect of Chronic Administration of Safranal on Systolic Blood Pressure in Rats. Iran J Pharm Res. 2015; 14: 585–590. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25901167
- Lahlou S, Interaminense Lde F, Leal-Cardoso JH, Morais SM, Duarte GP. Cardiovascular effects of the essential oil of Ocimum gratissimum leaves in rats: role of the autonomic nervous system. Clin Exp Pharmacol Physiol. 2004; 31: 219–225. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15053817
- el Tahir KE, Ashour MM, al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. Gen Pharmacol. 1993; 24: 1123–1131. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8270171
- Bigliani MC, Rossetti V, Grondona E, Lo Presti S, Paglini PM, et al. Chemical compositions and properties of Schinus areira L. essential oil on airway inflammation and cardiovascular system of mice and rabbits. Food Chem Toxicol. 2012; 50: 2282–2288. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22546367
- I Menezes IA, Barreto CM, Antoniolli AR, Santos MR, de Sousa DP. Hypotensive Activity of Terpenes Found in Essential Oils. Z Naturforsch C J Biosci. 2010; 65: 562–566. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21138056
- Kim DS, Goo YM, Cho J, Lee J, Lee DY, et al. Effect of Volatile Organic Chemicals in Chrysanthemum indicum Linné on Blood Pressure and Electroencephalogram. Molecules. 2018; 23: 2063. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30126122
- Bahrami T, Rejeh N, Heravi-Karimooi M, Vaismoradi M, Tadrisi SD, et al. Effect of aromatherapy massage on anxiety, depression, and physiologic parameters in older patients with the acute coronary syndrome: A randomized clinical trial. Int J Nurs Pract. 2017; 2: e12601. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29071755
- Hosseini S, Heydari A, Vakili M, Moghadam S, Tazyky S. Effect of lavender essence inhalation on the level of anxiety and blood cortisol in candidates for open-heart surgery. Iran J Nurs Midwifery Res. 2016; 21: 397–401. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27563324
- Salamati A, Mashouf S, Mojab F. Effect of Inhalation of Lavender Essential Oil on Vital Signs in Open Heart Surgery ICU. Iran J Pharm Res. 2017; 16: 404-409. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28496494
- Iokawa K, Kohzuki M, Sone T, Ebihara S. Effect of olfactory stimulation with essential oils on cardiovascular reactivity during the moving beans task in stroke patients with anxiety. Complement Ther Med. 2018; 36: 20–24. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29458924
- Esmaeili H, Sharifi M, Esmailidehaj M, Rezvani ME, Hafizibarjin Z. Vasodilatory effect of asafoetida essential oil on rat aorta rings: The role of nitric oxide, prostacyclin, and calcium channels. Phytomedicine. 2017; 36: 88–94. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29157833
- Soares MC, Damiani CE, Moreira CM, Stefanon I, Vassallo DV. Eucalyptol, an essential oil, reduces contractile activity in rat cardiac muscle. Braz J Med Biol Res. 2005; 38: 453–461. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15761626
- Bordia A. Effect of garlic on blood lipids in patients with coronary heart disease. Am J Clin Nutr. 1981; 34: 2100–2103. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7293938
- Bordia A, Bansal HC, Arora SK, Singh SV. Effect of the essential oils of garlic and onion on alimentary hyperlipemia. Atherosclerosis. 1975; 21: 15–19. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1131298
- Ismail. The Functional Effect of Kaempferia Parviflora on Ischemic Stroke in Rats. Am J Agricultural Biological Sci. 2012; 7: 173–179.
- Brook RD, Glazewski L, Rajagopalan S, Bard RL. Hypertension and triglyceride catabolism: implications for the hemodynamic model of the metabolic syndrome. J Am Coll Nutr. 2003; 22: 290–295. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12897043
- Gilani SN, Khan AU, Gilani AH. Pharmacological basis for the medicinal use of Zanthoxylum armatum in gut, airways and cardiovascular disorders. Phytother Res. 2010; 24: 553–558. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20041426
- Grassmann J, Hippeli S, Spitzenberger R, Elstner EF. The monoterpene terpinolene from the oil of Pinus mugo L. in concert with alpha-tocopherol and betacarotene effectively prevents oxidation of LDL. Phytomedicine. 2005; 12: 416– 423. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16008117
- Hernández JJ, Ragone MI, Bonazzola P, Bandoni AL, Consolini AE. Antitussive, antispasmodic, bronchodilating and cardiac inotropic effects of the essential oil from Blepharocalyx salicifolius leaves. J Ethnopharmacol. 2018; 210: 107–117. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28811222
- Chen HW, Wei BJ, He XH, Liu Y, Wang J. Chemical Components and Cardiovascular Activities of Valeriana spp. Evid Based Complement Alternat Med. 2015; 2015: 1–11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26788113
- Ziaee M, Khorrami A, Ebrahimi M, Nourafcan H, Amiraslanzadeh M, et al. Cardioprotective Effects of Essential Oil of Lavandula angustifolia on Isoproterenol-induced Acute Myocardial Infarction in Rat. Iran J Pharm Res. 2015; 14: 279–289. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25561934
- Maiwulanjiang M, Chen J, Xin G, Gong AG, Miernisha A, et al. The volatile oil of Nardostachyos Radix et Rhizoma inhibits the oxidative stress-induced cell injury via reactive oxygen species scavenging and Akt activation in H9c2 cardiomyocyte. J Ethnopharmacol. 2014; 153: 491–498. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24632018
- Occhiuto F, Circosta C. Cardiovascular Properties of the Non-Volatile Total Residue from the Essential Oil of Citrus bergamia. Int J Pharmacognosy. 1996; 34: 128–133.
- Lahlou S, Figueiredo AF, Magalhães PJ, Leal-Cardoso JH, Gloria PD. Cardiovascular effects of methyleugenol, a natural constituent of many plant essential oils, in normotensive rats. Life Sci. 2004; 74: 2401–2412. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14998717
- Zhang Q, Fan K, Wang P, Yu J, Liu R, et al. Carvacrol induces the apoptosis of pulmonary artery smooth muscle cells under hypoxia. Eur J Pharmacol. 2016; 770: 134–146. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26607464
- McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 1999; 52: 465–477. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10416955
- Tan W, Li Y, Wang Y, Zhang Z, Wang T, et al. Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions. Biomed Pharmacother. 2017; 90: 244–252. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28363170
- Durić K, Kovac Besovic EE, Niksic H, Muratovic S, Sofic E. Anticoagulant activity of some Artemisia dracunculus leaf extracts. Bosn J Basic Med Sci. 2015; 15: 9-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26042507
- Occhiuto F, Circosta C. Cardiovascular Properties of the Non-Volatile Total Residue from the Essential Oil of Citrus bergamia. Int J Pharmacognosy. 1996; 34: 128–133.
- Kim JH, Lee HJ, Jeong SJ, Lee MH, Kim SH. Essential oil of Pinus koraiensis leaves exerts antihyperlipidemic effects via up-regulation of low-density lipoprotein receptor and inhibition of acyl-coenzyme A: cholesterol acyltransferase. Phytother Res. 2012; 26: 1314–1319. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22275303
- Lorenzo PS, Rubio MC, Medina JH, Adler-Graschinsky E. Involvement of monoamine oxidase and noradrenaline uptake in the positive chronotropic effects of apigenin in rat atria. Eur J Pharmacol. 1996; 312: 203–207. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8894597
- Whitaker EJ, Pham K, Feik D, Rams TE, Barnett ML, et al. Effect of an essential oil-containing antiseptic mouthrinse on induction of platelet aggregation by oral bacteria in vitro. J Clin Periodontol. 2000; 27: 370–373. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10847543
- Lai L. Orally Administered Essential Oil Composition and Use Thereof. 2017.
- Oleszek W. Method for extracting flavonoids from horse chestnuts. 2011.
- Jodlbauer HDD. Nutritional supplement with germinated perilla seed. 2005.
- Holzer A. Compositions and methods for treating heart disease. 2018.